Structure of Zeolite
The Structure of Zeolite
what makes Zeolite so unique is its rigid structure, which is arranged in an ordered, matrix configuration resembling a bee's honeycomb. The microscopic channels, cavities, pores, cells or cages (we wilcall them cages as they trap molecules), which form this intricate honecomb structure, are usually uniform in shape and size. As the Zeolites are microporous crystalline solids, they have a well-defined geometric framework (honeycombs) such as the two shown below.
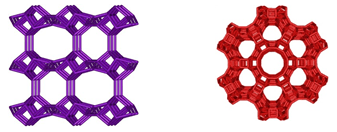
A defining feature of all Zeolites is that they are insoluble and are composed of a stable combination of silicon, aluminum and oxygen in their framesworks. Most Zeolites are made up of 4-connected networks of atoms, called a tetrahedral. A tetrahedral is formed with a silicon atom in the middle and oxygen atoms at the corners.
The tetrabedral can then be linked together by their corners (see illustration below) to from a variety of beautiful structures. The framework structure may contain the linked cages, which are of the right size to allow smaller molecules to entre ie. the limiting cage sizes are roughly between 3 and 10 Å in diameter.
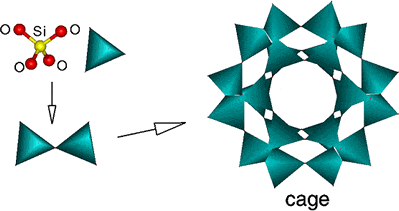
This is due to a very regular pore structure of the cages molecular dimensions. The maximum size of the molecular or cation that can enter the cages of a Zeolite is controlled by the diameters of the cages. These are conventionally defined by the ring size of the aperture, where, for example, the term '8-ring' refers to a closed loop that is built from eight tetrahedral co-ordinated silicon (or aluminum) atoms and eight oxygen atoms.
These rings are not always perfectly flat and symmetrical due to a variety of effects, including pressures during formation and the strain induced by the bonding between unites that are needed to produce the overall structure, or co-ordination of some of the oxyen atoms of the rings to cations within the structre. Therefore, the cage openings are not always identical in natural Zeolites and that gives them their great versatility in dealing with many different molecules.
